Abstract
Barth syndrome (BTHS; OMIM# 302060) is an X-linked disorder of neutropenia, cardiomyopathy and muscular weakness due to mitochondrial dysfunction secondary to inherited tafazzin (TAZ) mutations. Neutropenia occurs in the majority of BTHS patients, leading to high risk of recurrent life-threatening bacterial infections. The etiology of the G-CSF-responsive neutropenia in BTHS is incompletely understood and appears to reflect impaired granulopoiesis. Long-term risk of myelodysplasia (MDS) in BTHS individuals requiring chronic G-CSF therapy is unknown due to the rarity of the disorder; further, genotype-phenotype correlations between the severity of neutropenia and TAZ mutations remain to be elucidated. Genetic Taz knockout is embryonic lethal in the mouse. Therefore, a genetic BTHS mouse model is urgently needed to improve our mechanistic understanding of this disease and develop clinical studies based on preclinical evidence.
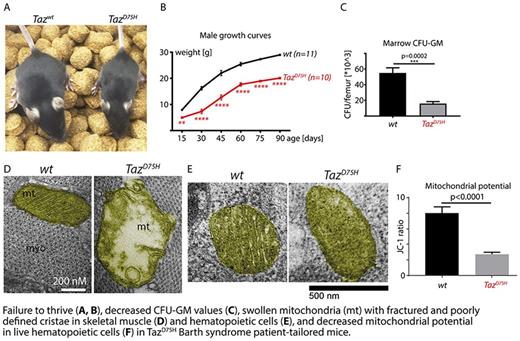
We employed CRISPR/Cas system to edit our BTHS patient's tafazzin point mutation (D75H) into the endogenous murine Taz gene. The resulting mice recapitulated hematopoietic and systemic manifestations of Barth syndrome. TazD75H males are born at less than expected Mendelian frequencies (p<0.0001) and suffer from perinatal lethality and failure to thrive with 69% decrease of body size at postnatal day 29 compared to age-matched wt males (p<0.0001). TazD75H males have neutropenia at 1 month of age evidenced by complete blood counts and independent manual peripheral smear analysis (p=0.04). Young TazD75H mice had mildly decreased bone marrow cellularity per femur (p=0.01675) accompanied by deficient functional bone marrow colony-forming ability compared to age- and sex-matched wt mice (approximately 3.5-fold decrease in CFU-GM, p=0.0002, and CFU-GEMM, p=0.0041), consistent with a functional hematopoietic defect. Similar to BTHS patients, TazD75H mice develop prenatal cardiomyopathy, with echocardiograms of surviving mice demonstrating left ventricular non-compaction (p=0.0339) and decreased ejection fraction (p=0.038). Further, TazD75H mice recapitulate the Barth syndrome phenotype of impaired skeletal muscle strength as shown by decreased ability to lift weights and perform hanging exercise (p=0.0072). Interestingly, TazD75H males developed infertility due to profound spermatogenesis arrest (p<0.0001). These data indicate that TazD75H mice recapitulate all crucial clinical findings in Barth syndrome.
Given the role of tafazzin in mitochondrial cardiolipin metabolism, we examined the structure and function of mitochondria in TazD75H mice. Strikingly, transmission electron microscopy revealed accumulation of swollen mitochondria with fractured cristae in TazD75H hematopoietic cells, cardiomyocytes and skeletal muscle, reflecting the mitochondrial phenotype of BTHS patients. Total mitochondrial mass quantified by MitoTracker flow was unchanged in TazD75H hematopoietic cells, but flow cytometry using the JC-1 mitochondrial membrane potential probe revealed disrupted mitochondrial potential during live TazD75H hematopoiesis in multi-potential progenitors (MPPs, p=0.0025), Lin- Sca-1+ c-kit+ cells (p<0.0001), Lin- Sca-1- c-kit- (p=0.0036), lineage-negative (p<0.0001) and lineage-positive (p=0.0211) hematopoietic cells. Quantitative Seahorse metabolic profiling revealed impaired mitochondrial respiration in live TazD75H hematopoietic cells, confirming functional mitochondrial deficiency.
In summary, our work demonstrates that impaired hematopoiesis in Barth syndrome, which is embryonic lethal in the mouse, can be recapitulated in vivo through editing a patient-specific hypomorphic tafazzin mutation into the murine genome. The precision-medicine TazD75H mouse model recapitulates clinical hallmarks of BTHS in hematopoietic and non-hematopoietic tissues, accompanied by structural and functional mitochondrial defects. This work enables in vivo studies to enhance our mechanistic understanding and treatment of hematopoetic and other defects in Barth syndrome.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.